In the definition of desert from different perspectives the most important definitions are as follows. From the point of view of climatology, desert is a land that average annual rainfall is less than 50 mm and does not rain during the year or years. And even if it rains it does not reach the ground and the total rainfall capacity for many years rain within a few days or a few hours and the annual evaporation rate is about 100 times the annual rainfall. Such as the desert of Qatar, Saudi Arabia’s Rub ‘al Khali desert, the desert of Sahara in Africa, which can be divided into two parts.
One of the extremely dry deserts with an annual rainfall of 20 to 50 mm, and the second is over-dry deserts with less than 20 mm of rainfall per year. If the rainfall is between 50 and 100 mm, it is considered as a semi-desert region. Desertification is considered as the third most important challenge of the world in the twenty-first century. According to reports from international organizations One sixth of the population, three-quarters of dry land and one third of the worlds land an area of five billion hectares in 110 countries, it is exposed to the phenomenon of desertification.
Threats to the destruction of 73% of the world’s total rangelands with an area of 3.3 billion hectares, reducing soil production capacity in 47 percent of the world’s arid regions, unusable 50 to 70 thousand square kilometer of fertile land per year and over 42 billion dollars in annual damage to agricultural products, with its vast and widespread ecological, Social, economic and environmental effects. Particularly widespread poverty and the destruction of basic resources considered as part of the desertification effects. At present, natural factors do not play a significant role in desertification. Of course, the droughts that occur will increase desertification but human activities are more likely to result in desertification, and the most important factor is population growth. On a large scale, many studies have shown that the occurrence of dust storms in very dry areas with no vegetation cover is about 60-80 days per year, areas covered with bush plants is 30 to 20 days a year and then the grasslands area is 2-4 days a year. Meng and Lu showed that people living in non-industrial areas of China, but close to areas with high dust, 3 to 6 days after dust due to upper respiratory tract infection, lung inflammation and high blood pressure there are more daily visits to the hospital.
Dust particles by reducing the activity of the superoxide dismutase enzymes and the level of Glutathione in the lungs and the liver as well as increasing the level of TBARS (as an indicator of lipid peroxidation) in lungs, heart and liver, cause oxidative damages in these organs. Particles smaller than 2.5 microns increase the permeability of the plasmid membrane and increases the level of intracellular calcium and leads to cytoplasmic toxicity. The most common eye diseases among citizens are Pterygium and sandy keratitis (Sand particles cause the cornea to be scratched and reduce vision). More than a billion people in the world are threatened by desertification.
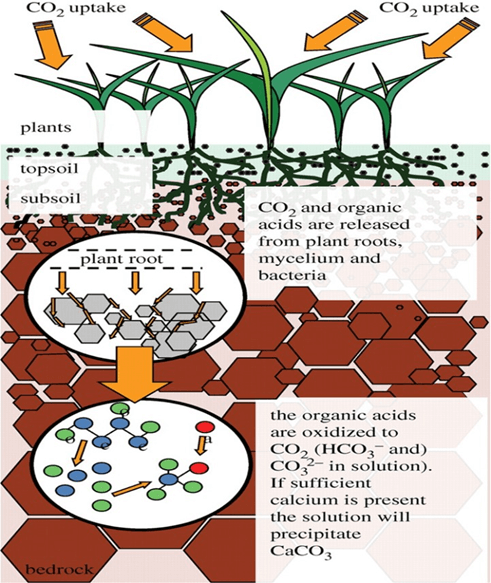
During the process of photosynthesis, plants absorb atmosphere carbon dioxide to provide their needed organic materials. In recent years, it has been proven that plants can precipitate carbonate in the soil through absorbing carbon dioxide during photosynthesis and reaction of the roots with soil.
Carbonate precipitates stabilize the soil over the years. It is known that sand dunes and deserts are stabilized through arboriculture. The tree’s roots transact with soil around rhizosphere which results in carbonate production in soil and increased soil adhesion, so that stabilization is maintained in the sand dunes even after plants destruction. In addition to microorganisms, calcium carbonate can be produced by chemical mechanisms in nature.
Plants roots secrete anions of organic acids of low molecular weight such as acetate, malate, oxalate, and citrate. Organic acids anions are unstable and decompose quickly, giving rise to a combination of dissolved carbon dioxide and bicarbonate which are equilibrated and finally form carbonate.
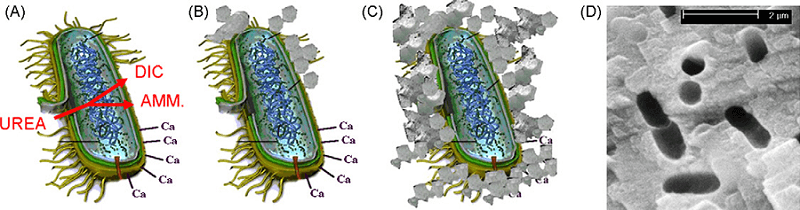
In recent years, a relatively green and sustainable soil improvement technique has been introduced which is called microbiologically induced calcite precipitation (MICP, same mechanism exploited by BIO-STROMATECH). This technique uses biochemical processes to improve soil engineering properties (such as strength and permeability).
As a research field related to different sciences, MICP locates at the intersection of microbiology, geochemistry, and civil engineering, and plays a role in related applications including waste water treatment and restoration of limestone.
Many studies have been performed so far to investigate the precipitation of calcium carbonate through microbiological induction (MICP) in relation to reinforcement of soil, surface reconstruction, brick, self-healing of conventional concrete, and reconstruction of historical monuments.
To boost the plant growing exploiting the ground stabilization process, we invented and developed BIO-STROMATECH, a product capable to grow plants everywhere in the world, even in the harshest desertic areas.